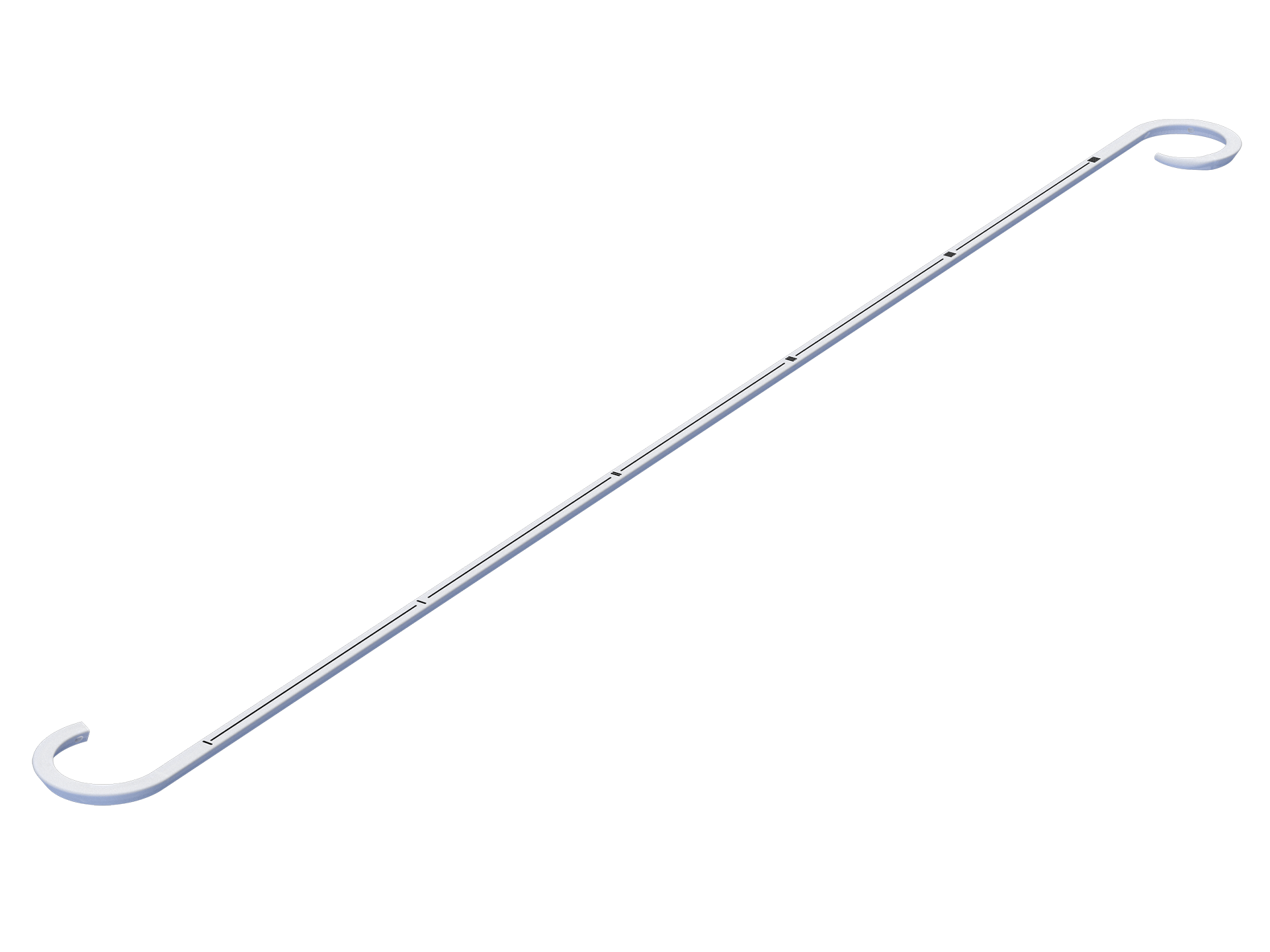
The Stone Basket is used to endoscopically capture, manipulate, release and remove stones and other foreign bodies in the upper urinary tract (ureter, renal pelvis, and calices) during urological surgery.
This device is intended for use in endoscopic removal of ureteral and renal stones.
Features:
---Kink resistant Nickel-titanium alloy inner core with precise advancement and reflection feel.
---Zebra stripes for easier endoscope observation.
---PTFE coating that is easy to be lubricated
---Prevent stone migration during the lithotripsy.
---Increase stone fragmentation efficiency.
---Simple design for swift operation.
---CE MDR FDA certified.
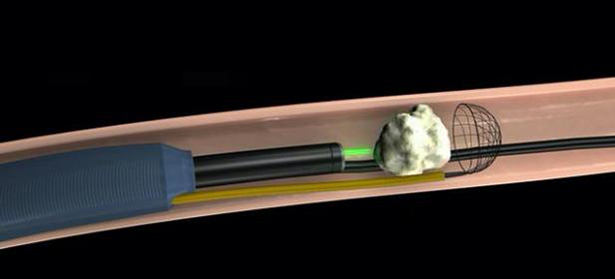
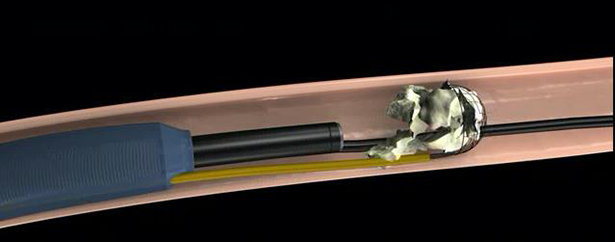
Working Mechanism: (Reference photos, the real coil is different)
Step 1: stop the stone's migration for laser fragementation
Step 2: retrieval the stone fragments to the sheath
Include but not limited to the patients who are (or have) pregnant, kidney malignancy, pyohemia, anticoagulant or abnormal bleeding, severe ureter stenosis, severe infections of the urinary tract and prostatic hyperplasia.
-Please read this instruction carefully before using;
-This product should only be used by the trained surgeons or nurses.
-This product does not contain hydrophilic coating;
-It is FORBIDDEN to be used if there is package damage, product damage, or the product expires when checking in accordance with the "Use Method 1";
-This product is sterilized by ethylene oxide, only for one-time use, the validity of sterilization is three years. Do not use the product if the package is damaged or exceeds the validity period;
-When using the product, the medical staff should gently push and pull the product to avoid too large a range of motion leading to product bending;
-When the basket enters working channel of the endoscope, ensure that the basket is in a closed state;
-In the process of using this product to retrieve stones, in the event of retrieving larger stones stuck, stop the operation immediately, operate the control handle to close the basket, and withdraw it from the body;
-Dispose of the product after using in accordance with the rubbish management regulation of medical device.
Ordering Information
No. | Model | Basket type | Basket Opening Width(W ) | Basket length(B) | Maximum sheath diameter(ΦC) | Working length(L) | optional accessories |
1 | 211T10A0 | T type | 10mm | 10mm | 0.8mm | 110cm | / |
2 | 211T10A1 | 10mm | 10mm | 0.8mm | 110cm | Spin lock adapter | |
3 | 211T10B0 | 10mm | 10mm | 0.8mm | 115cm | / | |
4 | 211T10B1 | 10mm | 10mm | 0.8mm | 115cm | Spin lock adapter | |
5 | 211T10C0 | 10mm | 10mm | 0.8mm | 125cm | / | |
6 | 211T10C1 | 10mm | 10mm | 0.8mm | 125cm | Spin lock adapter | |
7 | 211T10D0 | 10mm | 10mm | 0.8mm | 123cm | / | |
8 | 211T10D1 | 10mm | 10mm | 0.8mm | 123cm | Spin lock adapter | |
9 | 211F08A0 | F type | 8mm | 18mm | 0.8mm | 110cm | / |
10 | 211F08A1 | 8mm | 18mm | 0.8mm | 110cm | Spin lock adapter | |
11 | 211F08B0 | 8mm | 18mm | 0.8mm | 115cm | / | |
12 | 211F08B1 | 8mm | 18mm | 0.8mm | 115cm | Spin lock adapter | |
13 | 211F08C0 | 8mm | 18mm | 0.8mm | 125cm | / | |
14 | 211F08C1 | 8mm | 18mm | 0.8mm | 125cm | Spin lock adapter | |
15 | 211F08D0 | 8mm | 18mm | 0.8mm | 123cm | / | |
16 | 211F08D1 | 8mm | 18mm | 0.8mm | 123cm | Spin lock adapter | |
17 | 222A05E0 | A type | 5mm | 6mm | 1.2mm | 120cm | / |
18 | 222A05F0 | 5mm | 6mm | 1.2mm | 130cm | / | |
19 | 222A08E0 | 8mm | 6mm | 1.2mm | 120cm | / | |
20 | 222A08F0 | 8mm | 6mm | 1.2mm | 130cm | / | |
21 | 222A10E0 | 10mm | 6mm | 1.2mm | 120cm | / | |
22 | 222A10F0 | 10mm | 6mm | 1.2mm | 130cm | / | |
23 | 222C05E0 | C type | 5mm | 6mm | 1.2mm | 120cm | / |
24 | 222C05F0 | 5mm | 6mm | 1.2mm | 130cm | / | |
25 | 222C08E0 | 8mm | 6mm | 1.2mm | 120cm | / | |
26 | 222C08F0 | 8mm | 6mm | 1.2mm | 130cm | / | |
27 | 222C10E0 | 10mm | 6mm | 1.2mm | 120cm | / | |
28 | 222C10F0 | 10mm | 6mm | 1.2mm | 130cm | / |
Demonstration video:
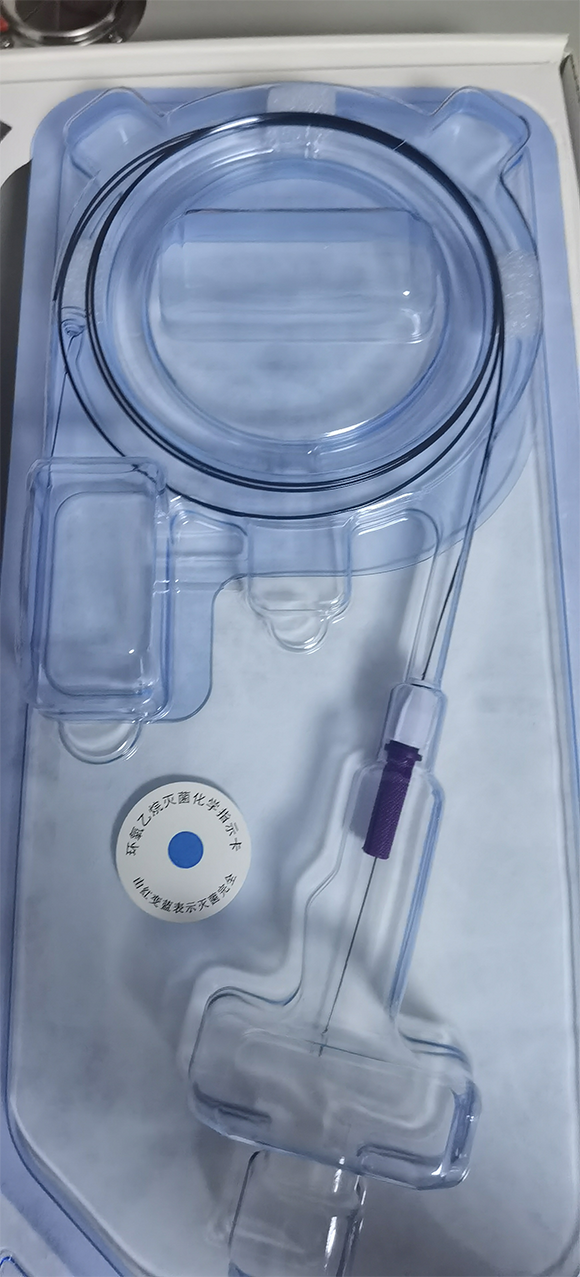
Real Package Photo:
Address: Rm 102-2, Longtian Tongfuyu Ind Estate #11-3, and Rm 401 & 402, Bldg D3, YingZhan S&T Estate, Longtian Tongfuyu Rd #8; Longtian Com, Longtian St, Pingshan, Shenzhen, GD, China
Telephone: 0086-755-82927901
Email: sales@triousmed.com
Website: www.triousmed.com